The choice to validate equipment in a laboratory or pharmaceutical testing facility can be crucial to the success or failure of the trials being carried out. The fundamental purpose of laboratory water system validation is to provide assurances that the system is suitable for its intended use. The assurance is supported by documented evidence that the system consistently performs according to predetermined specifications for its intended application. At Veolia Water Technologies UK, we have several solutions with validation available.
What is Validation?
In pharmaceutical, medical and clinical industries, validation is carried out to provide quality assurance that a product or process is fit for purpose and will consistently deliver results as expected. This is a crucial factor of laboratory testing, as any inconsistency in formulation or method may affect the test or product outcome. To maintain Good Laboratory Practice (GLP), the validation process is crucial.
Key Components of Water System Validation
According to the FDA's Process Validation Guidance, validation consists of three main stages:
Stage 1 – Process Design
- Establishing critical process parameters
- Determining quality attributes
- Identifying potential failure modes
- Using risk assessment tools to understand process variables
Stage 2 – Process Qualification
- Design of facility and qualification of equipment/utilities
- Process Performance Qualification (PPQ), which:
- Confirms facility, utilities, and equipment operate as intended
- Demonstrates the commercial process performs as expected
- Establishes documented evidence
Stage 3 – Continued Process Verification
- Routine monitoring of quality attributes
- Collection and evaluation of data trends
- Maintaining the process in a state of control
- Detecting unplanned departures from the process
Solutions and Implementation
Purelab Chorus Systems
The PURELAB® Chorus Lab Water System range is an ideal solution for customers in the biotech, pharmaceutical and testing laboratory sectors who require systems that are validation tested. Each product in the range has the option to include on-site validation with an engineer and an easy-to-use validation support manual. This provides a smooth step-by-step progression through the validation support process to guarantee successful qualification.
Validation Support and Documentation
The validation support manual provides documentary evidence to demonstrate that the chosen Chorus system is installed in accordance with IQ specifications and operates in accordance with the OQ system design criteria. The manual also includes certificates of conformity and calibration generated during manufacturing and the quality control process. Finally, we provide detailed documentation of all quality control and calibration testing carried out on a system prior to it leaving our manufacturing facility. This approach reduces the testing required to be carried out on site once installed and guarantees quality, allowing you to make an informed decision.
Ongoing Quality Assurance
We also provide an annual requalification service designed to provide the highest level of validation support for the lifetime of a water purification system. It provides assurance that a system is continuing to meet user requirements and is still providing the same high-quality water as it did at installation.
Product Range and Specifications
There are several solutions in the PURELAB® Chorus Water System range to suit different pure water requirements. The PURELAB® Chorus 1 is the ideal solution for applications that require the highest level of water purity as it benefits from our innovative PureSure® technology.
Systems fitted with PureSure® technology feature a double purification pack and monitoring system, which helps to ensure accurate results and uninterrupted workflow. In turn, the method enables guaranteed, optimum, pure water quality, as well as advanced warning of consumable change and extended consumable service life.

Alternatively, the PURELAB® Chorus 1 Complete is a ‘Tap to Type I’ system, which can provide 18.2mW ultra-pure laboratory water (Type I), at up to 20 litres an hour direct from a potable water supply. This water can then be dispensed either directly from the system or from a choice of Halo Dispensers, which can be connected in multiple locations throughout a facility without having to pay for additional systems.

Finally, for customers who only need Type II water, the PURELAB® Chorus 2 is also available, providing the same levels of reliability and convenience.
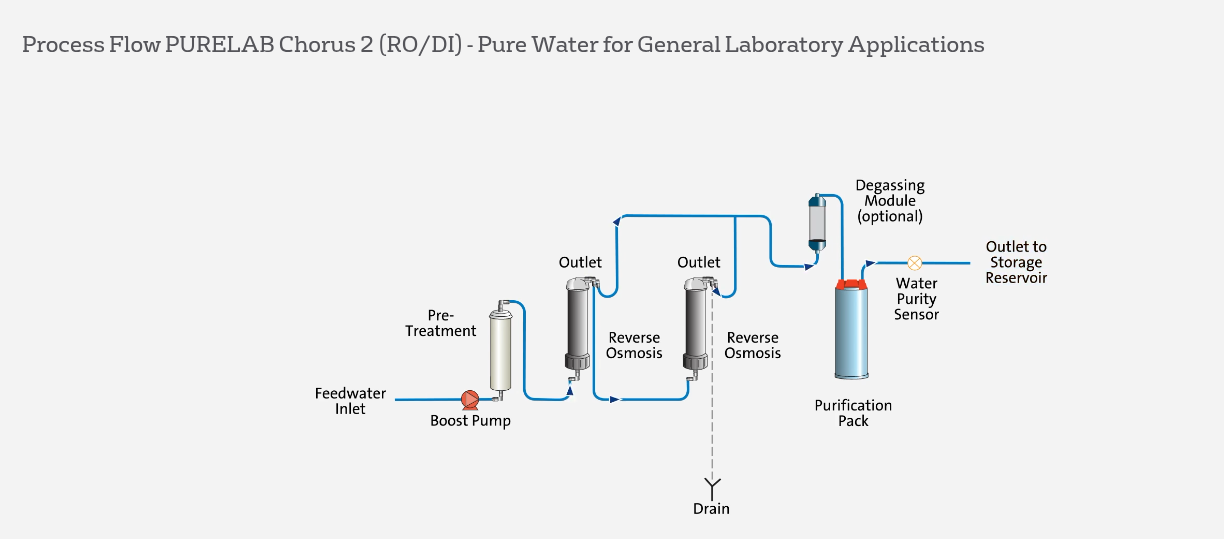
System Selection and Support
Selecting the right systems for a facility isn’t always easy. That is why we have recently launched our PURELAB® Selector Tool to make specifying the correct lab water System simple. By answering just a few questions, this application can quantify the suitability of the different range solutions based on your site’s specific needs and recommend the best product for you.
Once a system has been selected, the app then demonstrates how the chosen system will work and provides important information on the technologies that make it function.
For more information about the PURELAB® Selector Tool, please click here.