The healthcare sector is witnessing unprecedented growth in endoscopy procedures, driving the need for sophisticated sterile services facilities. While many focus solely on equipment selection, successful facility design requires a more comprehensive approach. Here's what healthcare facility planners need to know.
Understanding Modern Regulatory Requirements
Navigating the complex landscape of healthcare facility design starts with regulatory compliance. Before designing a new endoscope reprocessing unit, it is essential to check the relevant Health Technical Memorandum (HTM) and ISO guidelines. These regulations and guidelines form the foundation of any new endoscope reprocessing unit. As an example, ISO 15883 sets specific standards for washer-disinfectors and water quality, while ISO 13485 ensures robust quality management systems for medical devices. Meeting these requirements early in the design phase prevents costly modifications later.
Strategic Space Planning for Future Growth
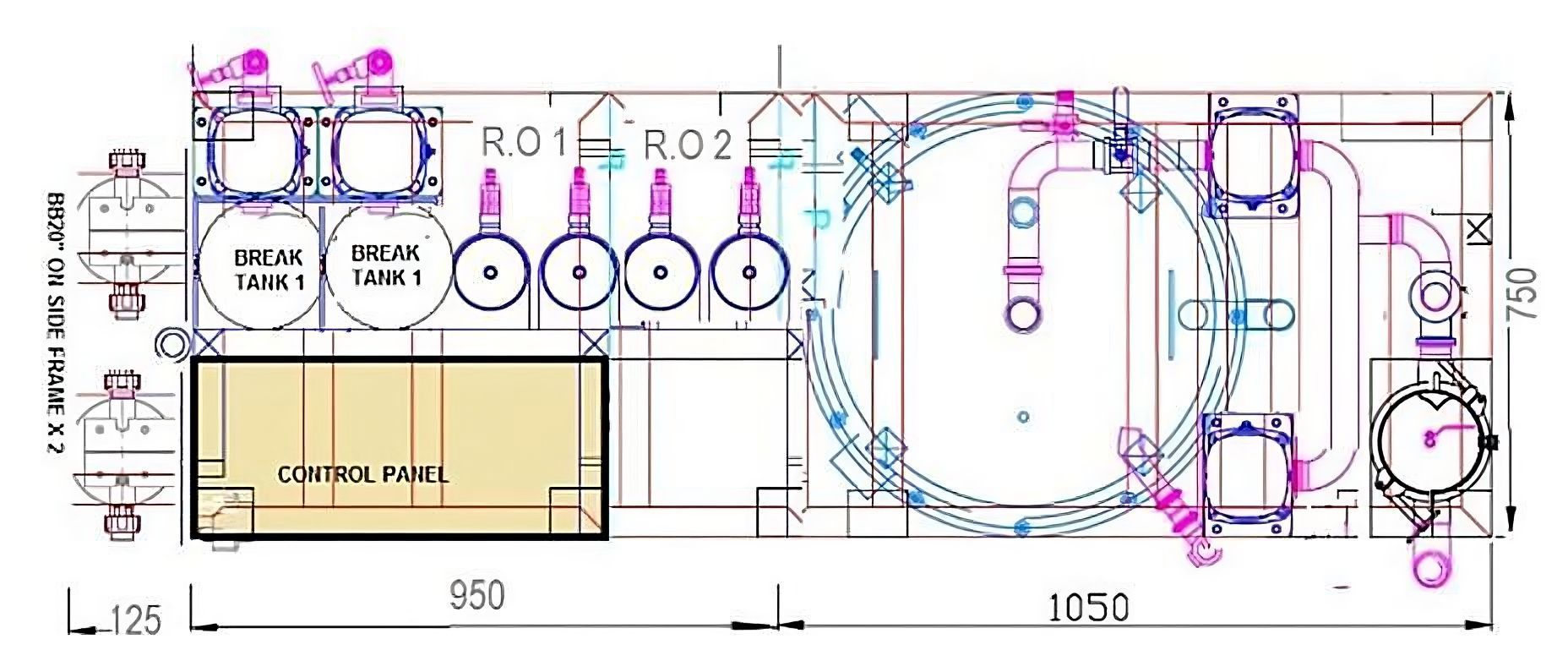
Today's facility must meet tomorrow's demands. Modern endoscopy units require careful consideration of patient throughput, not just for current operations but for anticipated growth over the next decade. This foresight influences decisions about automated endoscope reprocessors, washer-disinfectors, and sterilisers.
Water treatment infrastructure presents a particular challenge. The location of water treatment systems and storage facilities can significantly impact operational efficiency. Whether designing a new building or retrofitting an existing space, key questions must be addressed:
- Will the plant room accommodate future expansion?
- How will water quality be maintained throughout the system?
- Is there adequate ventilation for optimal performance?
Optimising Movement and Workflow
Effective facility design goes beyond equipment placement. The most successful facilities create intuitive workflow patterns that enhance efficiency while maintaining strict contamination control. Engineers need adequate access for maintenance, staff require clear pathways for daily operations, and the overall design must account for the safe movement of both people and equipment.
Critical Infrastructure Decisions
The backbone of any endoscopy facility lies in its infrastructure. Water quality, in particular, can make or break facility performance. Whether sourcing from mains, ground, or well water, the chosen supply must undergo appropriate pre-treatment and purification through reverse osmosis. This isn't just about meeting standards – it's about protecting expensive equipment investments and ensuring optimal patient outcomes.
Modern facilities also demand sophisticated HVAC systems with precise environmental controls and robust electrical infrastructure to support increasingly complex equipment needs. These systems must work in harmony to maintain the controlled environment essential for sterile services.
Key Considerations for Success
- Prioritise water quality systems as foundational infrastructure
- Design for scalability in patient throughput
- Ensure compliance with current and anticipated regulations
- Create efficient workflows that maintain sterile barriers
- Plan for future technological integration
Healthcare facilities represent significant long-term investments. By carefully considering these essential elements during the design phase, organisations can create endoscopy and sterile service facilities that deliver value well into the future.
For more information on the regulatory guidelines and best practices relating to endoscopy reprocessing and sterile services suite management, visit our dedicated Healthcare page.